Purifying bispecifics: Easier said than done
Published date: 10 June 2025
Chains, mispairs, and madness
You’ve spent months engineering a bispecific that binds precisely, folds correctly, and looks great on paper. The biology is solid, the in vitro data is clean, and the expression system is cooperating. Then comes the part no one wants to talk about at the start: purity.
Not just ‘lab notebook pure’. Not 'good enough for SDS-PAGE' pure. You know what we mean: clinical-batch, regulatory-ready, stability-tested, manufacturing-grade pure.
That’s where reality sets in. Unlike their monoclonal cousins, bispecific antibodies don’t clean up nicely. They carry all the promise of molecular innovation, and all the complexity of a protein folding headache.
Two binding domains, four times the headache
Structurally, bispecifics are as much compromise as innovation. Every format (knob-into-hole, dual-variable domains, CrossMabs, tandem scFvs) brings unique benefits and its own set of liabilities.
What’s often underappreciated, until you’re knee-deep in the process, is just how much the structure influences everything that comes after expression. From chain mispairing and unwanted homodimers to half-antibodies and free light chains, the impact is far-reaching.
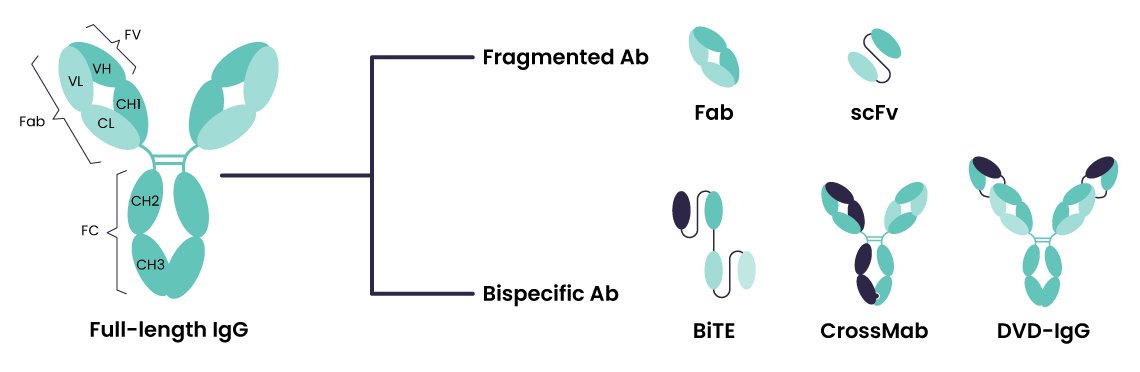
Figure 1. Antibody and bispecific antibody formats. Standard IgG (left) includes an Fc region, enabling Protein A purification and extended half-life. Bispecific formats (right) vary in structure and Fc content. Fragment-based formats like BiTEs (Bispecific T-cell Engagers) and diabodies lack Fc regions, requiring alternative purification strategies. Full-length formats such as knob-into-hole and CrossMab retain the Fc, allowing partial use of mAb purification platforms.
Now you’re downstream, and nothing works
If you’ve got an intact Fc-region, Protein A might give you a decent capture. But what comes off that column is a mixed population of mispaired species, aggregates, and fragments, all of which has to go.
Downstream purification for bispecifics isn’t just about yield and purity anymore. There are look-alike variants that mimic your target but aren’t, impurities that evade traditional polishing steps, and aggregates that disrupt immunogenicity profiles and spike SEC traces. It changes from format to format. What works well for one bispecific can fail completely on the next. A toolbox approach isn’t a luxury, it’s essential.
Platform processes gave us confidence with mAbs. We had the Protein A, ion exchange, and buffer exchange rhythm down to a tee. But bispecifics don’t fit that mold, and trying to force them into it means wasting time and losing product.
That’s why there is a shift from platform processes to precision strategies. Downstream scientists are having to act more like formulation chemists and molecular engineers, picking resins and conditions with surgical specificity. Fine-tuning pH gradients, tailoring salt steps, scouting ligands for the smallest charge or hydrophobic differences. It’s chromatography as a language, not just a tool.
Capture is just the start. Polishing is the minefield.
The first column is never the last fix. That’s where CEX, AEX, HIC, and mixed-mode resins show their value. The good ones aren’t just removing HCPs or DNA, they’re untangling variants.
Take charge variants, for instance. Mispaired bispecifics and aggregates often have different isoelectric points or surface charge profiles. Fine-tuning a salt gradient or pH window in a cation exchange step can deliver remarkable resolution, cutting aggregates from 20% to <5% while leaving your product intact.
Mixed-mode options bring another layer of finesse. You’re not only binding on charge or hydrophobicity—you’re exploiting both. That flexibility allows for wiggle room when standard resins plateau.
But it only works if you’ve done your homework, screening, and DOE. That’s how you get those clean SEC profiles and the measurable drop in host cell protein levels.
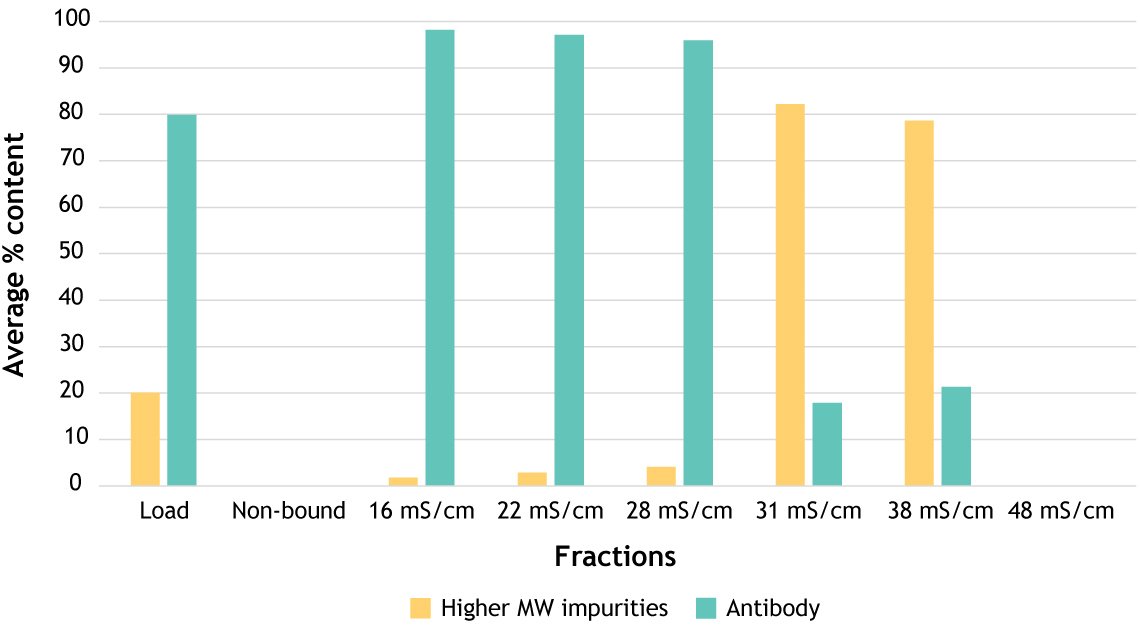
Figure 2. Using SP PuraBead® Edge for BsAb aggregate reduction (post-Protein A bispecific feed), reducing from 20% to <5% using an optimized step elution. Average percentage of higher molecular weight (MW) impurities (yellow) and antibody (teal) content determined by fluorescence SEC analysis. Graph shows the calculated percentage content for the load, non-bound, and each elution step fraction.
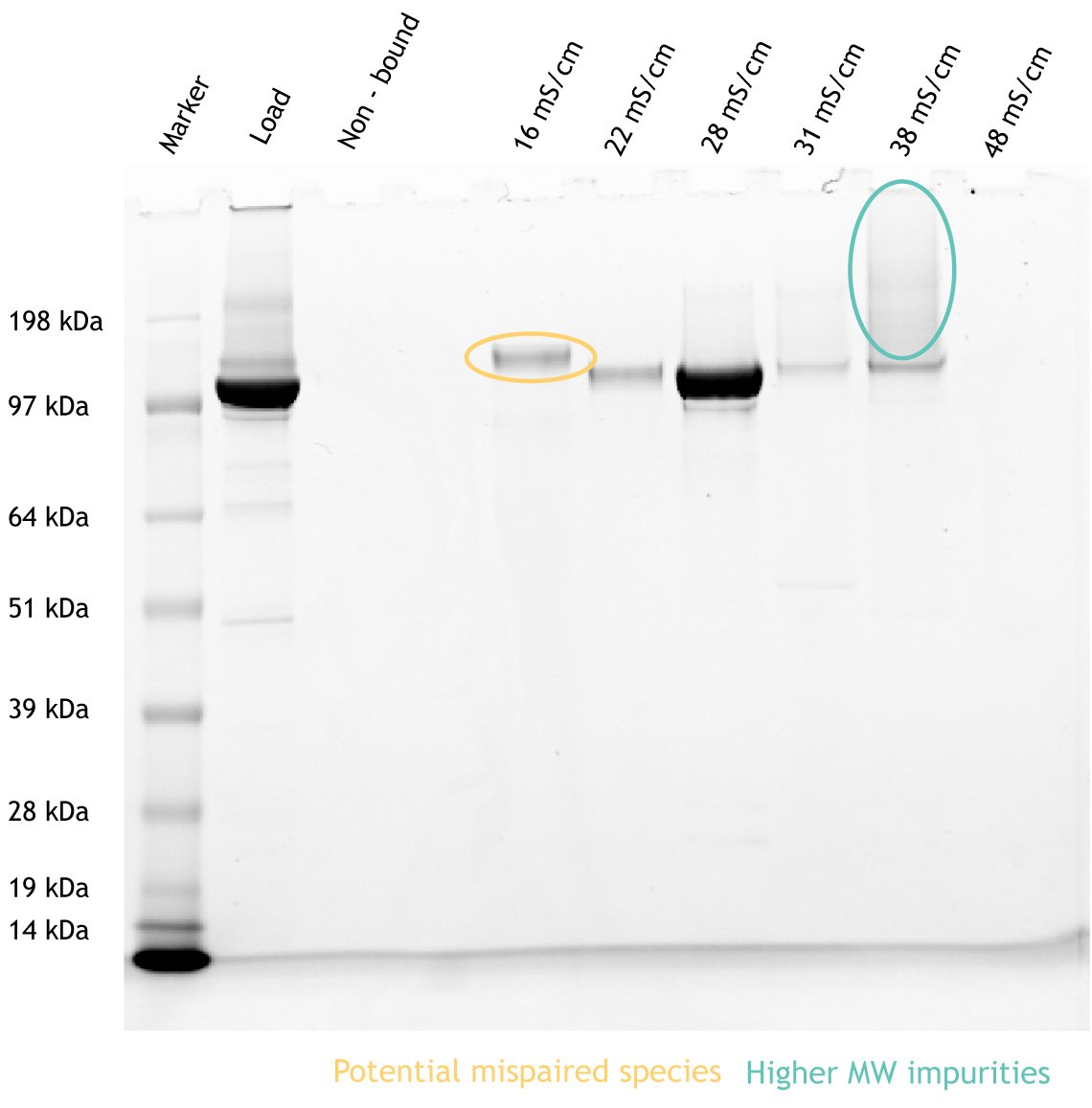
Figure 3. Post-polishing with SP PuraBead® Edge demonstrating the separation of unwanted impurities. SDS-PAGE analysis of the load, non-bound, and each elution step fraction. Band circled in yellow represents potential mispaired species. Banding circled in teal represents higher molecular weight (MW) impurities. The main target product is seen in the 28 mS/cm elution fraction at approximately 125 kDa.
Let’s talk about fragments
Some formats drop the Fc altogether, taking Protein A off the table. Maybe you turn to Protein L, only to find it struggles with lambda chains, reacts poorly to pH shifts, and offers binding capacity that makes you reconsider your approach.
That’s where synthetic ligands and alternative capture resins come in, designed to bind both kappa and lambda, withstand alkaline conditions, and elute gently enough to keep your fragments intact. They may not be platform, but they are versatile. And when you’re working with Fabs or scFvs that struggle to stay in solution, versatility makes all the difference.
What synthetic ligands offer, beyond performance, is operational resilience. No risk of immunogenic leachables, tough enough to handle harsh cleaning without compromising integrity, and performance that holds steady across dozens of cycles. In a world where every fragment format behaves differently, you need reliability built in. Not just a resin that works, but one that keeps working.
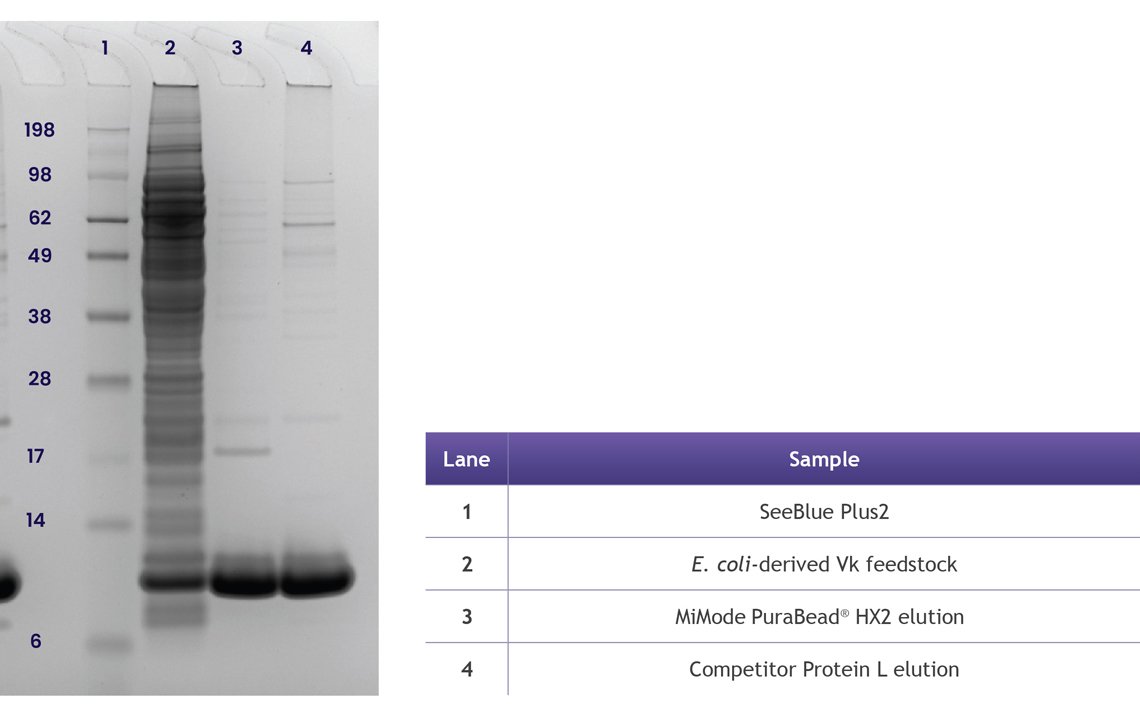
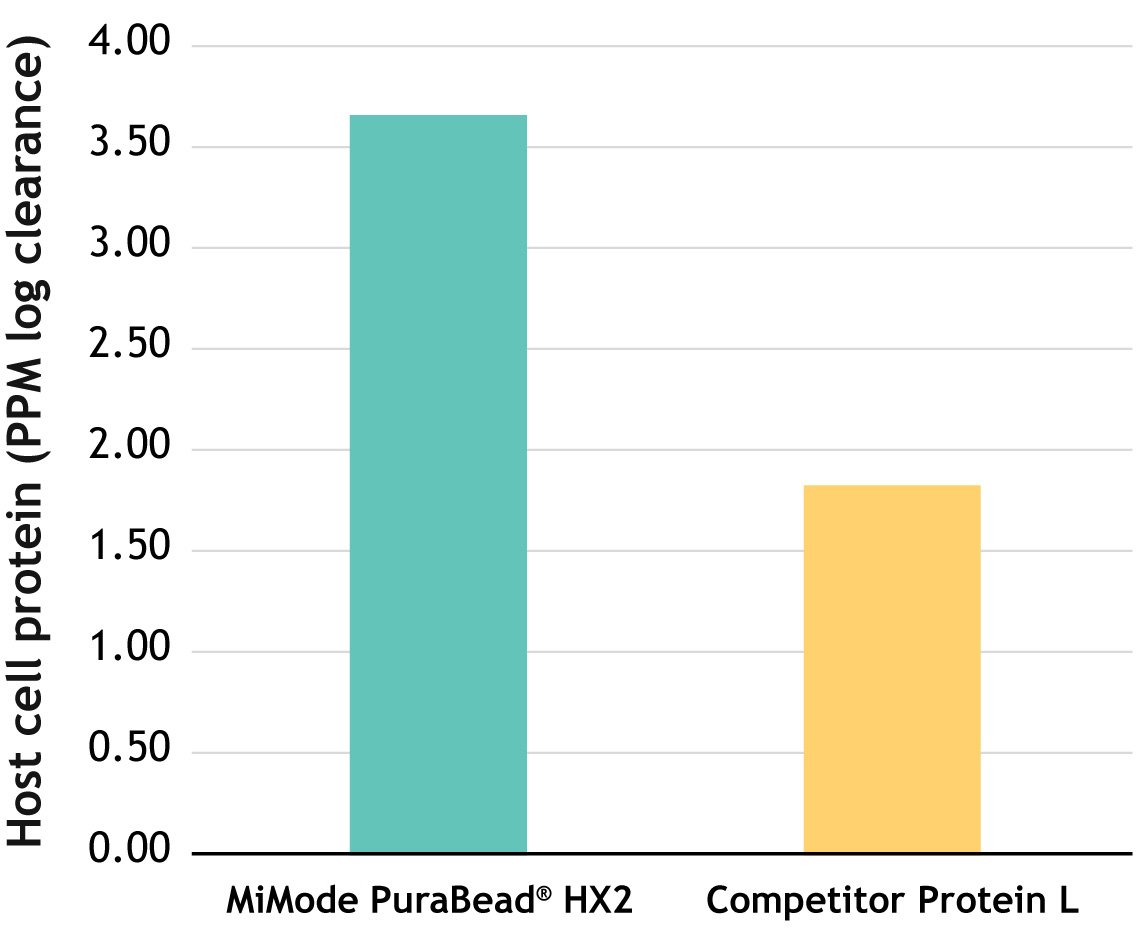
Figure 4. Competitor analysis of MiMode PuraBead® HX2 vs. a market leading Protein L resin showing comparable recovery and improved impurity profile on a model protein. An optimized purification workflow using MiMode PuraBead® HX2 achieved significantly higher host cell protein (HCP) clearance from an E. coli-derived Vk fragment feedstock compared to a Protein L resin (3.67 vs. 1.84 log PPM clearance). Elution recoveries were comparable (~77–78%), but MiMode PuraBead® HX2 delivered cleaner SDS-PAGE profiles and superior purity at a milder elution pH (5.0 vs. 2.5), reducing the risk of product denaturation and aggregation.
Process isn’t just a hurdle. It’s the gatekeeper.
You can have the most promising bispecific on paper, but if the process is fragile, inconsistent, or doesn’t scale, it won’t make it beyond Phase I. That’s the reality.
What happens in the column doesn’t stay there. It impacts long-term stability, and yield losses now become cost-of-goods discussions later. Process robustness isn’t just a nice-to-have, it’s product viability.
A soft word before you go
It’s demanding work, no doubt. But it’s what moves breakthroughs from beyond the bench to the clinic, and eventually into an actual treatment in a vial.
“With ever growing interest in more complex novel multispecific, whole antibody, and antibody fragment therapeutics, greater challenges are being presented to the industry with regards to downstream purification. Astrea Bioseparations has developed unique products that can provide effective and differentiated solutions to challenges with these target purifications. These solutions include separation of mispaired species, fragment impurities, aggregate removal, and clearance of process related impurities such as host cell proteins, endogenous nucleic acid impurities, and endotoxin. These products come with the added advantages of being extremely caustic stable, excellent packing and flow characteristics, and manufactured with non-toxic and sustainable components.” —Chris Sadler, Astrea Bioseparations R&D Resin Product Development Lead
If you’re deep in bispecific development and frustrated by process constraints, it might be time to stop forcing things to fit and start designing around the molecule. Forget rigid platforms. This is about smart tools, flexible strategies, and close collaboration.
When you’re ready, get in touch. We’ll bring the resins. You bring the challenges.
Resins featured in this blog: MiMode PuraBead® HX2 & SP PuraBead® Edge
To find out more about our antibody toolbox solution, please reach out to us at sales@astrea-bio.com or visit our antibody webpage.
Request a sample today!
Experience the efficiency and reliability of these resins in your workflow. Whether you’re optimizing processes in research or manufacturing applications, our sample program allows you to evaluate performance in the context that matters most to you.
Request sample here
