Tailoring antibody purification for next-generation therapeutics
Published date: 17 December 2025
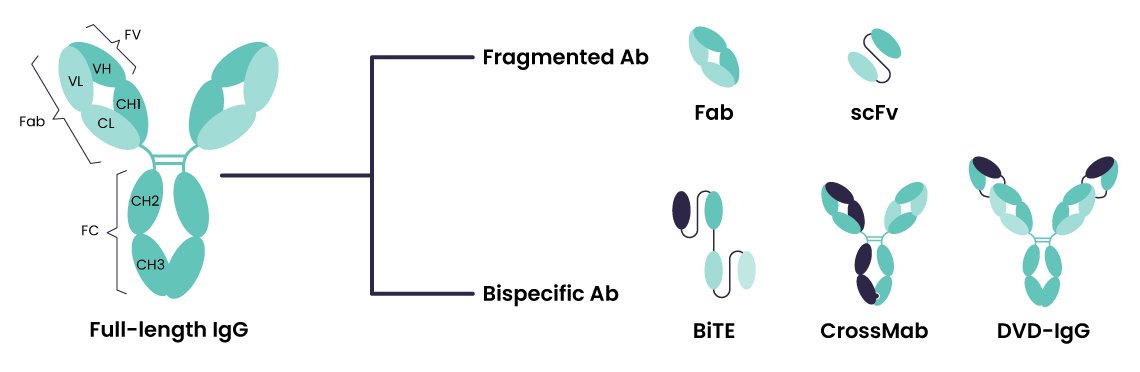
The antibody therapeutics landscape is quickly evolving. While traditional monoclonal IgGs have dominated the field for decades, the recent rise of engineered antibodies—including bispecifics, antibody-drug conjugates (ADCs), multispecifics, and other novel formats—is driving new expectations for downstream purification. This evolution presents enormous therapeutic potential, but also introduces new levels of complexity within downstream processing, particularly purification.
At a recent industry session, Maelia Uy-Gomez, Field Application Scientist at Astrea Bioseparations, shared insights on how our team is addressing these challenges with innovative chromatographic solutions tailored to modern antibody formats.
The challenge: Beyond “one-size-fits-all” purification
Every antibody behaves differently. Variations in size, charge, stability, and interactions with purification resins can significantly impact yield, purity, and process efficiency. While traditional Protein A chromatography has been a reliable solution for monoclonal IgGs, it falls short for next-generation constructs that lack the Fc region.
“Antibody purification can no longer rely on a one-size-fits-all workflow,” Maelia emphasized. “Each novel format introduces structural and biophysical complexities—mispaired chains, aggregation, and unpredictable impurity profiles—that require tailored solutions.”
Astrea Bioseparations’ purification toolbox: Flexibility meets performance
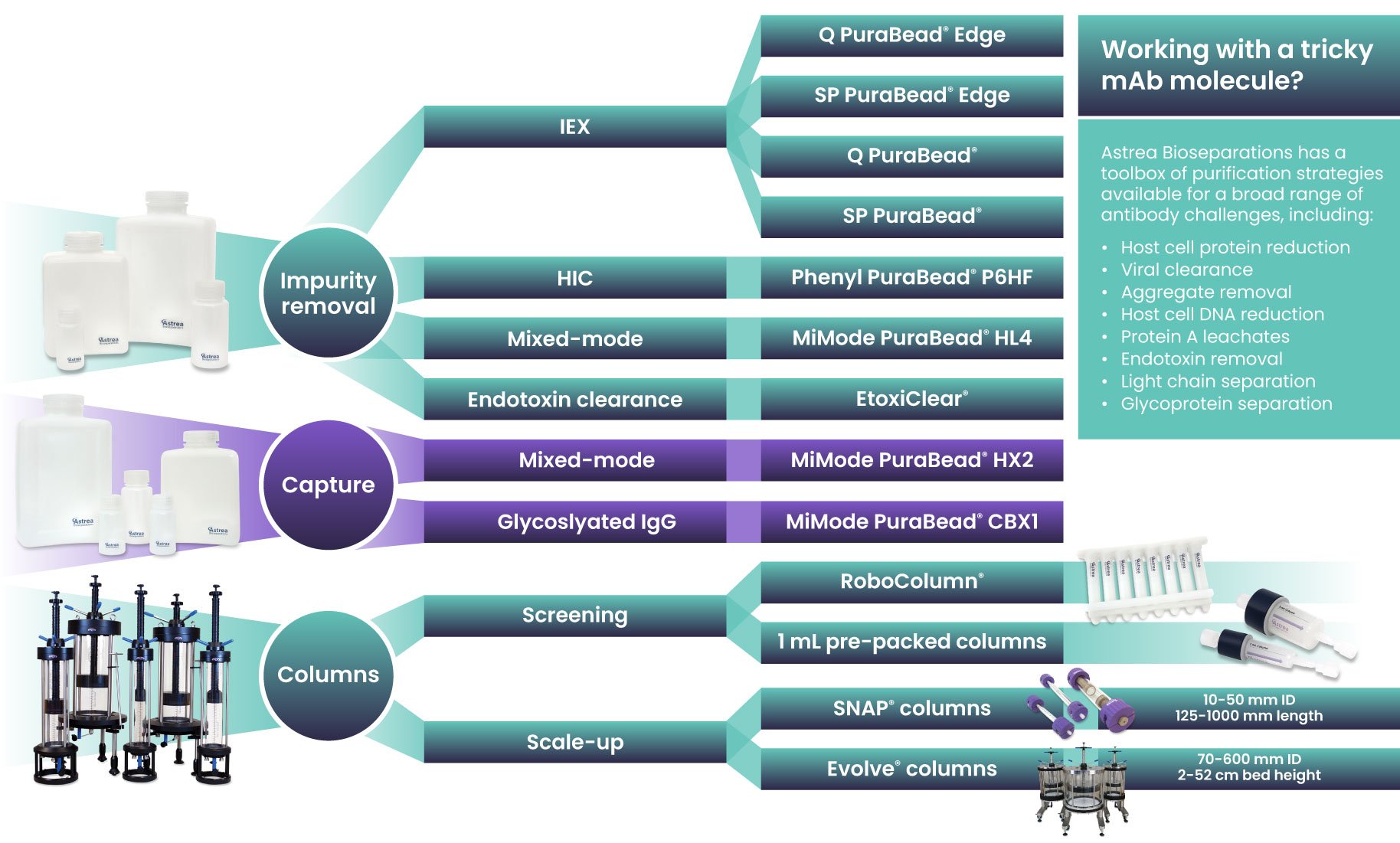
To meet these challenges, Astrea Bioseparations has developed a downstream purification toolbox that prioritizes flexibility, scalability, and high performance. This toolbox focuses on two key stages: capture and polishing, using format-specific resins across a range of chemistries, including mixed-mode, ion exchange, and high-resolution options.
- For Fc-lacking fragments: MiMode PuraBead® HX2, a mixed-mode capture resin, offers caustic stability and broad light chain affinity (Lambda and Kappa)
- For bispecifics and multispecifics: SP PuraBead® Edge, a next-generation strong ion exchange resin, balances high resolution with fast flow performance, ideal for structurally complex molecules
This toolbox approach supports high-throughput screening, lab-scale optimization, and GMP manufacturing, ensuring flexibility and consistency across every stage of development.
Case study 1: MiMode PuraBead® HX2 for Fc-free constructs
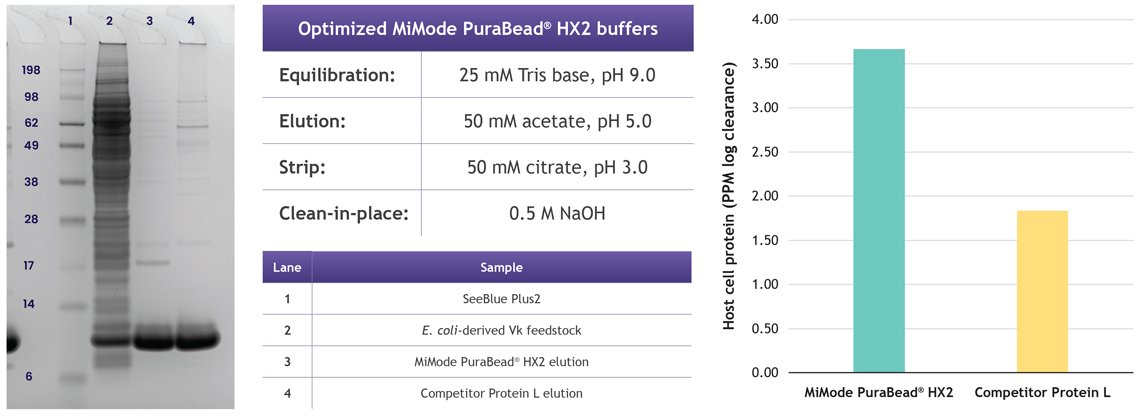
MiMode PuraBead® HX2 is designed for the purification of Fc-free antibodies that cannot be captured with Protein A. In a study with E. coli-derived Vk protein, key findings included:
- Load optimization: A DOE screen tested pH (7–10) and salt (0–0.5 M). Optimal performance was achieved at high pH and low salt, with ~15 mg/mL binding capacity and 80% recovery at >80% purity
- Elution optimization: Buffers including citrate, maleate, and acetate were assessed. Acetate at pH 5.5 provided the best balance of purity, host cell protein (HCP) clearance (3.5 log reduction), and mild conditions to minimize aggregation
- Performance comparison: Compared to Protein L, MiMode PuraBead® HX2 achieved comparable recovery (~77–78%) while providing significantly higher purity and lower risk of denaturation, highlighting its economic and operational advantages
Case study 2: SP PuraBead® Edge for bispecific antibodies
Bispecifics are prone to aggregation and mispaired chains, complicating purification. SP PuraBead® Edge, built on the ARO 65-micron base matrix, demonstrated:
- Superior flow properties and consistent dynamic binding capacity across residence times
- High-resolution separation, effectively resolving mispaired chains and aggregates while maintaining high throughput
- Scalability from early development to GMP, supporting robust platform processes
In a CHO-derived bispecific study, SP PuraBead® Edge reduced aggregate content from ~20% to under 5% and resolved six distinct species, illustrating its capacity for precise, tunable separations.

Sustainable performance with the PuraBead® base matrix
At the heart of these innovations is the PuraBead® base matrix, engineered in-house from sustainably sourced red seaweed. This platform provides:
- High mechanical stability and particle size control
- Low backpressure to support high-efficiency, scalable separations
- Biodegradability and excellent lifetime performance, with hundreds of clean-in-place (CIP) cycles
- Solvent-free manufacturing and reduced waste for more sustainable processing, and lower cost per batch
Technical performance and sustainability go hand-in-hand across the PuraBead® platform, delivering both environmental and economic benefits.
Key takeaways: Flexible purification solutions for a diversifying antibody landscape
With over 30 years of experience in bioprocessing, Astrea Bioseparations continues to innovate in antibody purification. Key insights from Maelia’s presentation include:
- The antibody landscape is diversifying—purification strategies must be adaptable
- Fc-lacking constructs, bispecifics, and complex modalities require tailored workflows
- MiMode PuraBead® HX2 and SP PuraBead® Edge demonstrate how format-specific resins improve yield, purity, and process efficiency
- Sustainable, scalable resin platforms support reliable, high-performance, and environmentally responsible bioprocessing—and remain central to Astrea Bioseparations’ approach
For researchers and developers tackling challenging antibody formats, Astrea Bioseparations’ toolbox offers the flexibility, control, and performance needed to streamline downstream workflows and optimize bioprocess outcomes.
To find out more about our antibody toolbox solution, please reach out to us at sales@astrea-bio.com or visit our antibody webpage.

