How nanofiber-based AEX purification is transforming manufacturing of lentiviral vectors and oncolytic viruses
Published date: 06 January 2026
As the advanced therapies industry accelerates, one challenge consistently rises to the top: How do we manufacture large, complex viral vectors at industrial scale without sacrificing purity, potency, or cost-efficiency?
Both lentiviral vectors (LVVs) and oncolytic viruses (OVs) are driving some of today’s most promising therapies, from CAR-T cell modification to cancer-selective viral immunotherapies. But their size, fragility, and biological complexity have strained traditional purification technologies.
This is where nanofiber-based anion exchange (AEX) chromatography is reshaping expectations.
A new weak AEX nanofiber platform, LentiHERO®, is demonstrating that high recovery, exceptional impurity clearance, and true scalability can be achieved together. Below is an overview of how this platform is redefining downstream purification across multiple viral modalities.
Why a new purification strategy is needed
The rise of LVVs, HSV-1, and vaccinia virus reflects a broader trend: Therapies are becoming larger and more complex.
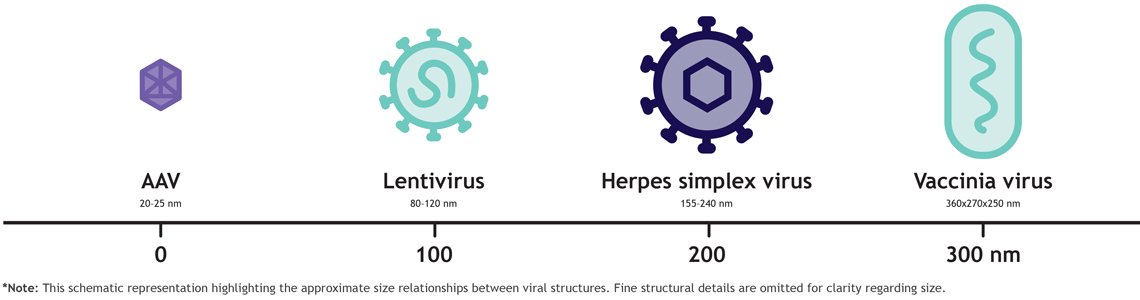
These large, enveloped particles are difficult to purify using packed-bed resins. Resin diffusion limits particle access, leading to low binding capacity, inconsistent recoveries, and poor impurity clearance.
And as manufacturing scales, these limitations only become more pronounced.
Nanofiber chromatography offers a fundamentally different approach. One built on open structures, convective flow, rapid mass transfer, and high capacity for large biomolecules and particles.
1. A high-capacity platform for VSV-G lentiviral vectors
In the first phase of the study, LentiHERO® demonstrated strong binding performance with VSV-G LVVs.
Its nanofiber structure allows viruses to interact directly with charged surfaces, without pore diffusion.
This results in:
- Higher dynamic binding capacity
- Faster processing times
- Reduced shear exposure
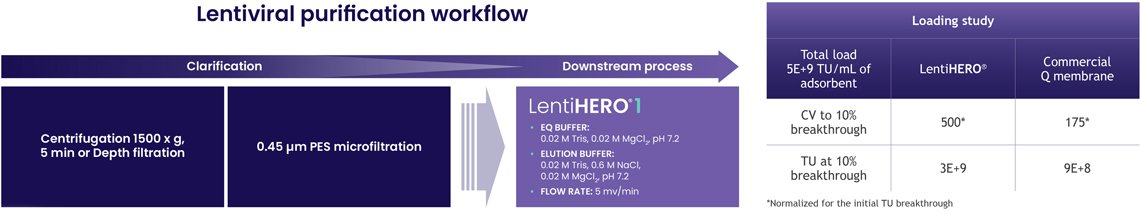
Together, these advantages form a strong foundation for scalable LVV manufacturing, an area where resin systems can often struggle without significant yield loss.
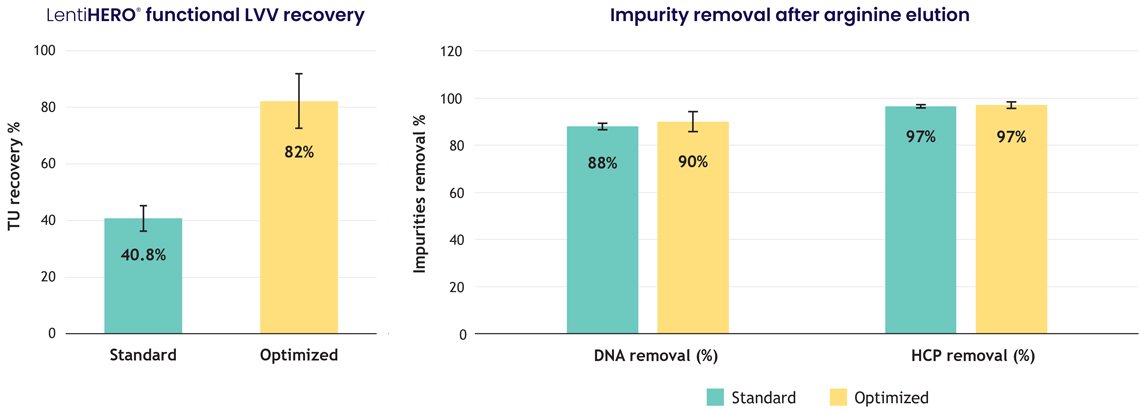
2. Optimized elution drives high yield and high purity
An optimized protocol, including arginine-based elution, enabled:
- 82% functional LVV recovery
- >2×10⁹ TU/mL of adsorbent
- >90% HCP and dsDNA clearance
This balance of high potency retention and deep impurity reduction is a key differentiator for nanofiber technology.
It supports regulatory expectations while preserving vector integrity.
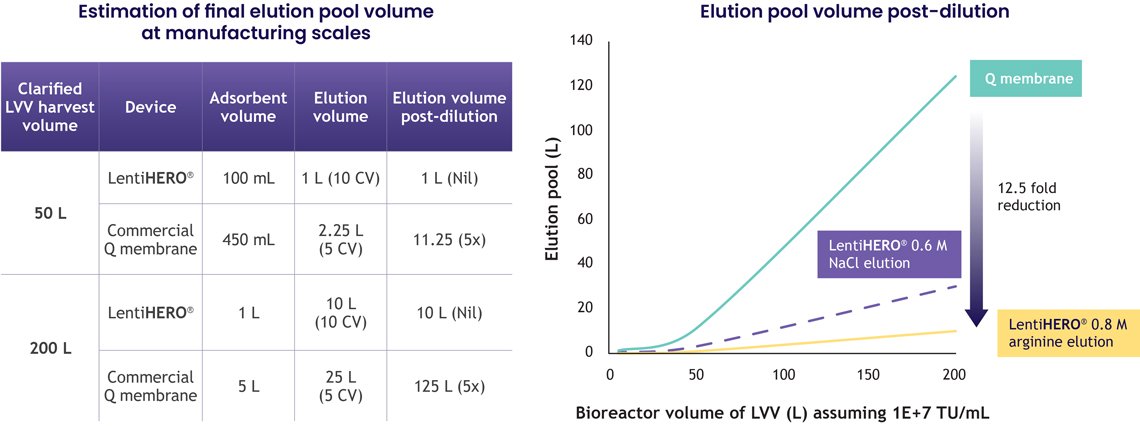
3. Lower volumes, lower buffer consumption, higher process efficiency
One meaningful advantage for manufacturers is a significant reduction in downstream volumes.
LentiHERO®’s elution strategy minimizes or eliminates:
- Post-elution dilution
- Extra conditioning steps
- Excess buffer consumption
At manufacturing scale, this drives:
- Smaller formulation tanks
- Faster turnaround
- Reduced facility footprint
- Lower cost per batch
For companies scaling from clinical to commercial production, these operational efficiencies have a direct impact on cost of goods sold (COGS).
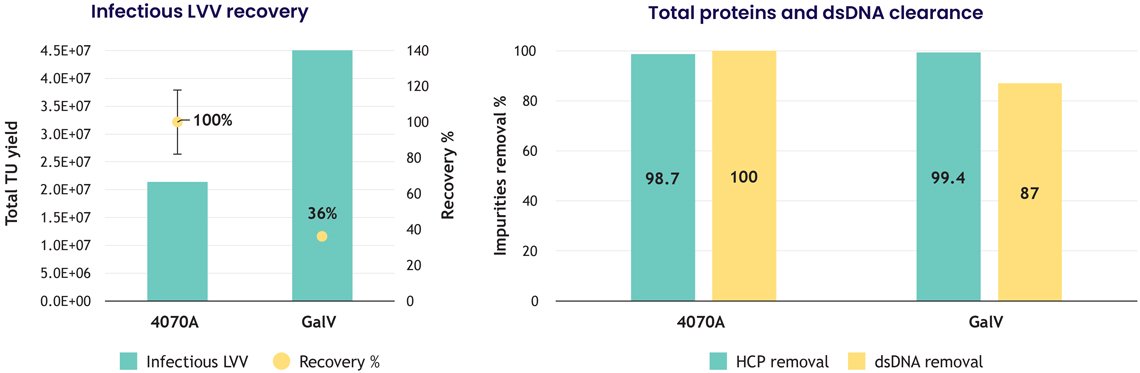
4. Purification across multiple LVV pseudotypes
Not all lentiviral vectors behave the same during purification. Envelope proteins influence surface charge and hydrophobicity, shaping interaction with chromatography media.
The study compared two pseudotypes with results showing broad applicability:
- 4070A: 100% (± 19%) recovery
- GalV: 36% recovery
Across both:
- >98% host cell protein removal
- >87% dsDNA reduction
This breadth makes the nanofiber platform valuable for teams working with next-generation LVVs.
5. End-to-end workflow for oncolytic viruses
Oncolytic viruses add further complexity due to large viral particles, multi-step harvest strategies, and high impurity loads.
The study evaluated a full upstream-to-downstream process, including:
- Infect Vero cells and monitor cytopathic effect
- Harvest lysate + supernatant at approximately day 3
- Clarify and filter (0.45 μm PES) after nuclease treatment
- Purify using LentiHERO® nanofiber AEX
- Quantify the viral recovery and impuritiesvia TCID₅₀, PicoGreen assay, and microBradford assay
This reflects the needs of industrial manufacturing: compatibility with real-world feed streams and heterogeneous impurity profiles.
6. High-purity, high-yield purification of HSV-1
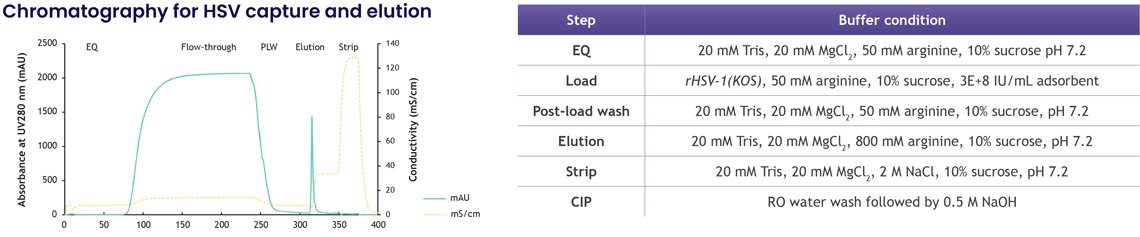
HSV-1, at nearly 200 nm, is one of the larger and more fragile particles in virotherapy. Achieving purification while maintaining structure is a significant challenge. LentiHERO® delivered:
- 73% HSV-1 recovery (1.4×10⁸ IU)
- 98% HCP clearance
- 75% dsDNA removal
TEM imaging confirmed structural integrity with intact envelopes, identifiable capsid geometry, and spherical morphology.
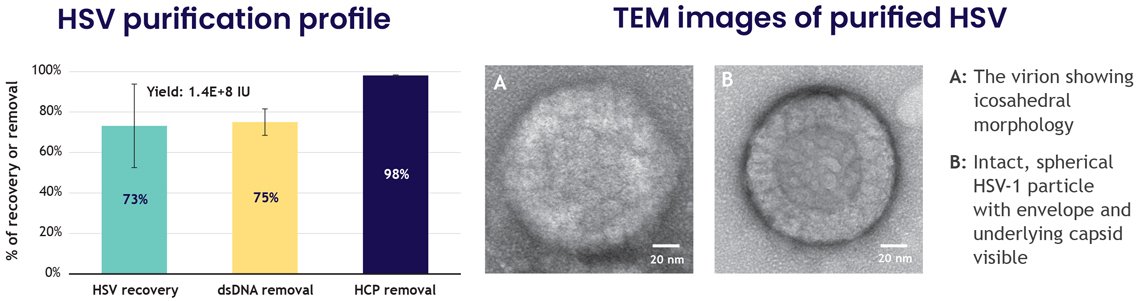
These results demonstrate that the nanofiber platform can handle even shear-sensitive, enveloped DNA viruses with confidence.
7. VACV: Purifying one of the largest therapeutic viruses
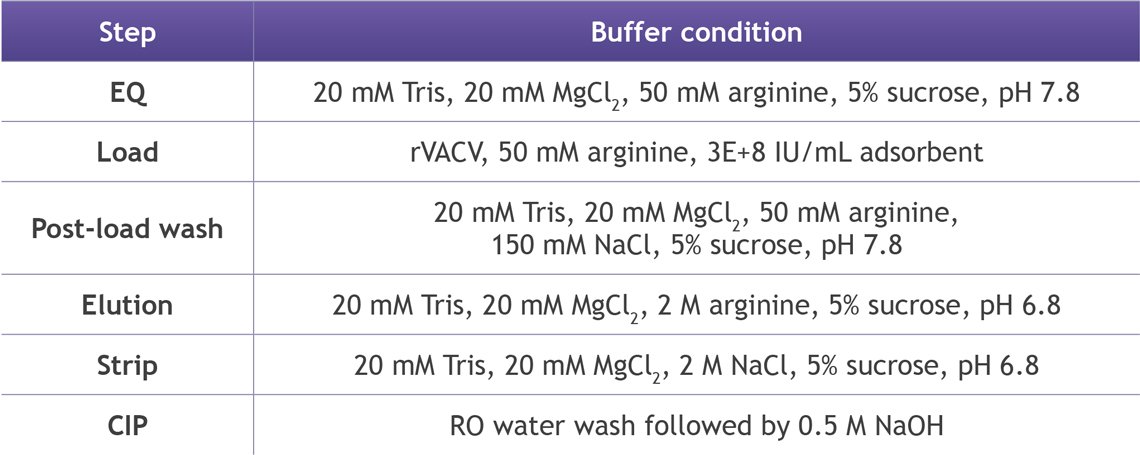
Vaccinia virus is among the largest clinically relevant particles and traditionally one of the most difficult to purify.
Despite its size and complexity, LentiHERO® achieved:
- 62% infectious VACV recovery (~1.7×10⁸ IU/mL adsorbent)
- >98% HCP removal
- >92% dsDNA clearance
TEM analysis showed well-preserved brick-shaped virions with dense internal cores, a visual confirmation of gentle handling.
This opens the door to downstream intensification for oncolytic platforms previously locked into low-yield legacy processes.
Conclusions: A versatile, scalable platform for the future of viral vector manufacturing
Across all viral systems tested — LVVs with multiple pseudotypes, HSV-1, and VACV — the nanofiber-based AEX platform delivered a compelling combination of yield, purity, scalability, and operational efficiency.
Performance at a glance:
- LVV recoveries: 36–100% (pseudotype-dependent)
- HSV-1 recovery: 73%
- VACV recovery: 62%
- HCP clearance: >97%
- dsDNA removal: 75–100%
- Elution volumes: Significantly reduced
- Processing: Faster, scalable, low-shear
Why it matters:
- Higher productivity per batch
- Streamlined tech transfer
- Predictable behavior across modalities
- Lower COGS for both clinical and commercial operations
- Compatibility with large, next-generation therapeutic viruses
What comes next:
The platform shows strong potential for expansion to:
- Adenovirus (Ad5)
- Virus-like particles (VLPs)
- Other large or non-enveloped viral systems
As the cell and gene therapy industry continues pushing toward larger-scale, more cost-efficient manufacturing, nanofiber-based purification stands out as a technology built for the future, not just the present.
Learn more about our lentivirus solutions with LentiHERO® here.

