Increasing Processing Efficiency, Purity and Recovery of Lentiviral Particles for Viral Vector Development
Published date: 12 December 2023
Therapeutic developers face significant challenges in purifying cell and gene therapy (CGT) modalities. Current processes rely on resin chromatography which was developed for monoclonal antibodies (mAbs) and are not fit for purpose for viral vector purification. Resin chromatography has significant limitations: Yields are low, processing times are long, and costs are high (Figure 1). To meet the global patient needs for affordable, life-changing therapies, developers require significant improvements over the current ill-suited methods. One way to meet those needs is with novel adsorbents that have considerably more binding surface area for the difficult-to-purify large molecules used in cell & gene therapies.
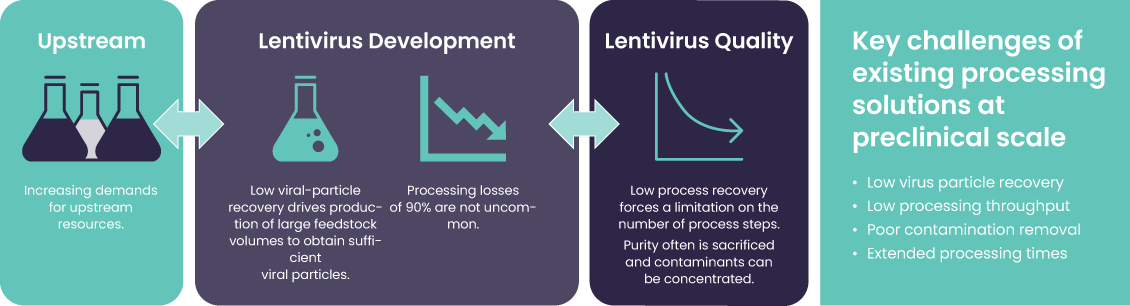
Figure 1: Legacy processing solutions place pressures on upstream production and lentiviral quality.
High binding surface area enables more effective processing and capture of lentiviral vectors
AstreAdept® nanofiber material is electrospun to form an open network with a large surface area that is highly accessible to viral vectors even at very fast flow rates, unlike traditional resin-based adsorbents (Figure 2). Astrea Bioseparations has incorporated the AstreAdept® technology into the easy-to-use Nereus LentiHERO® spin-column format to bring the advantages of functionalized nanofibers to viral vector purification at laboratory scale (Figure 3).
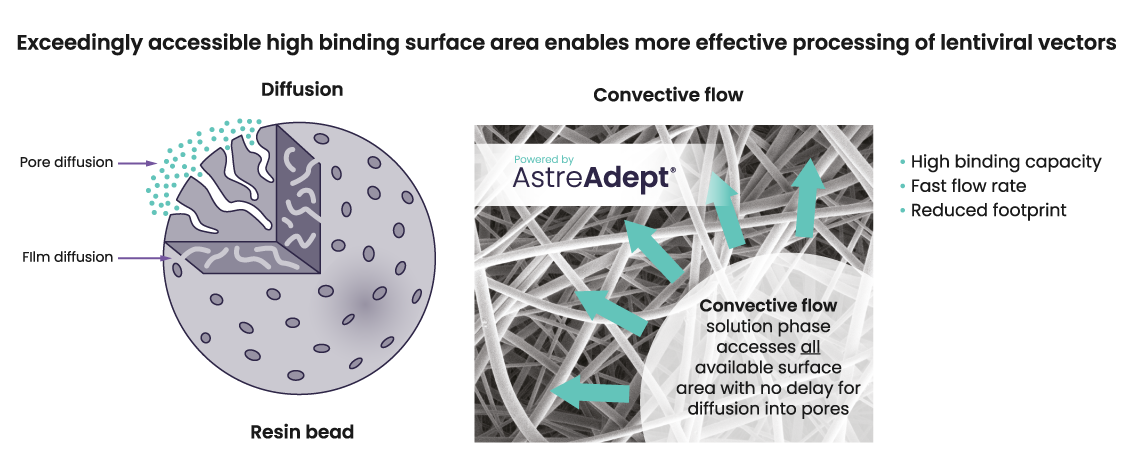
Figure 2: Immediate access to high binding surface area, with nanofiber technology, enables more effective processing of lentiviral vectors
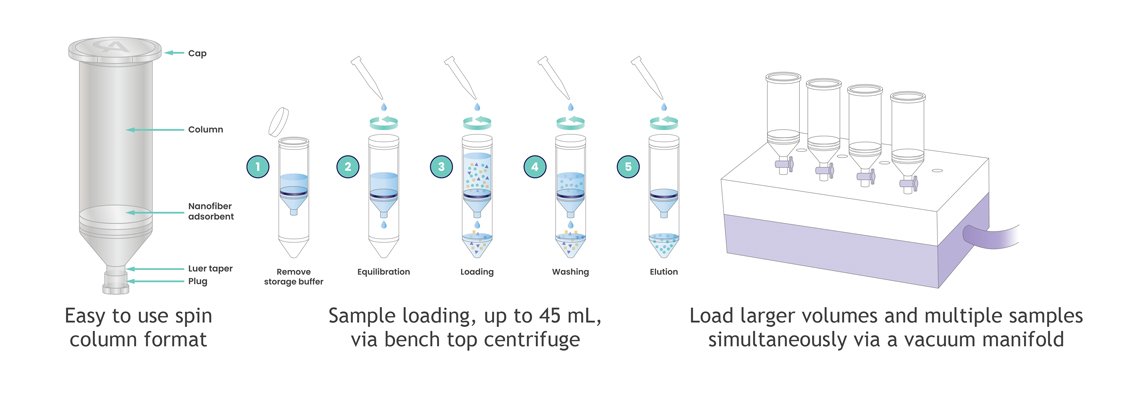
Figure 3: The LentiHERO® spin column is simple to use and scale-out, enabling increased throughput of LVV sample processing
High binding capacity and high recovery enables efficient lab-scale lentiviral processing
Increased lentiviral vector (LVV) capture is essential to maximizing LVV production for viral vector development. The Nereus LentiHERO® provides dynamic binding capacities of 4.7E+10 LVV particles per unit, enabling users to increase the amount of recovered materials from their current feedstock volumes and, in turn, reduces pressure to expand upstream production (Figure 4).
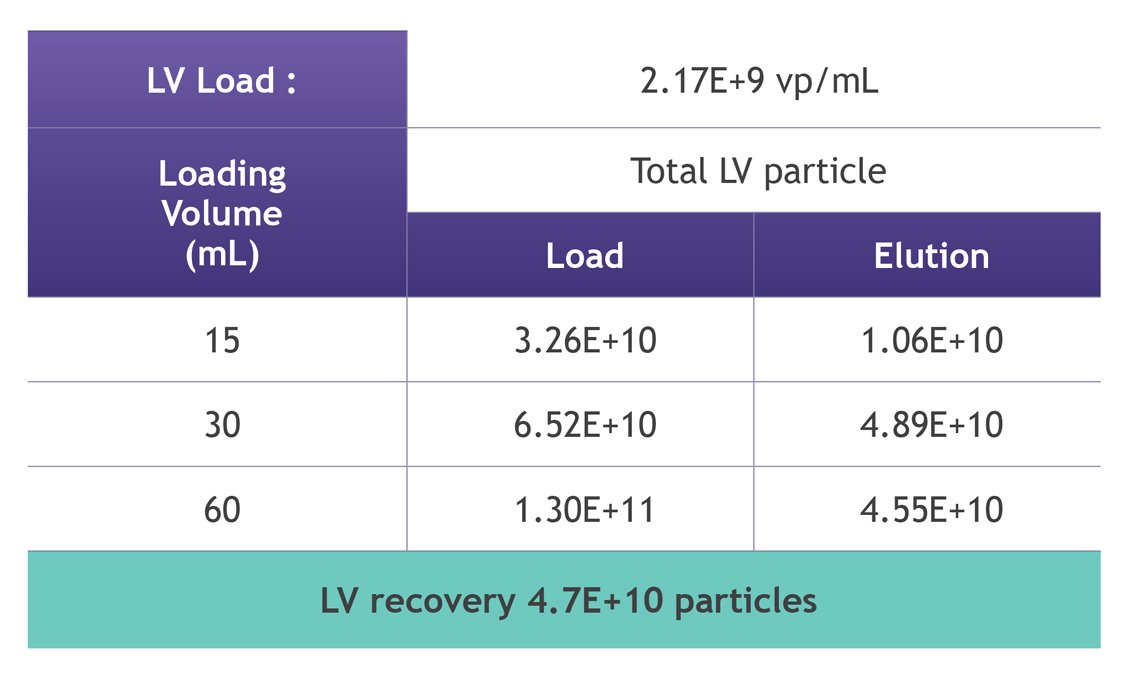
Figure 4: High LVV purification capacity per Nereus LentiHERO® column with varying LVV loads. Different volumes of LVV feedstock (produced from HEK293T cells) were loaded separately and purified following the technical user guide. Load and eluate samples were analysed by P24 ELISA to determine LVV physical titer
In addition to the superior capture characteristics of the underlying nanofiber technology, the Nereus LentiHERO® spin column enables gentle processing, including low centrifugal speed, reduced processing times, and mild elution conditions for high LVV recovery. This technology achieves yields of 60-80% of loaded LVV particles (Figure 5), which is a significant improvement over the 15–30% recoveries observed with other contemporary methods.
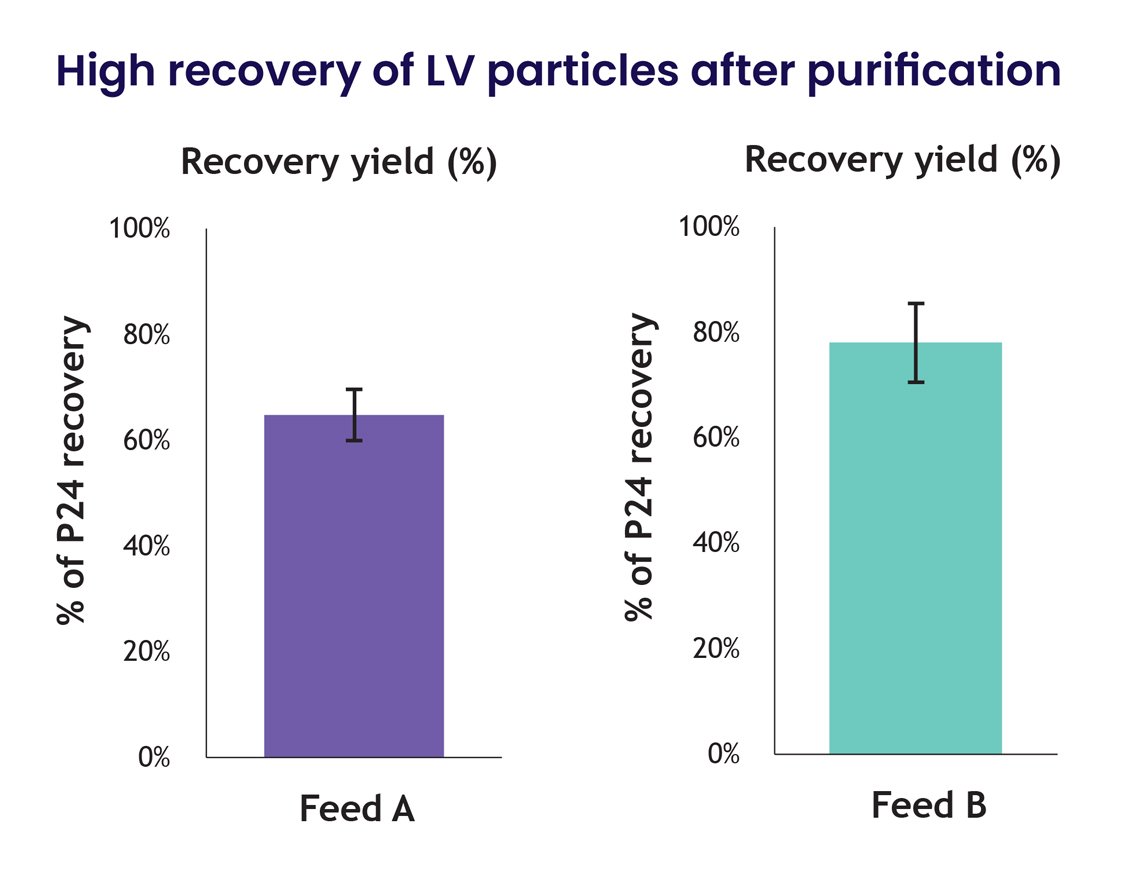
Figure 5: Feedstocks from 2 different external manufacturers (Feed A, Feed B) were independently purified and assayed (n=3/group). The total % of P24 recovery yield was relative to LVV input
Nereus LentiHERO® vastly decreases host cell proteins and DNA contamination
Minimizing host cell protein (HCP) and DNA contamination is important for limiting immunogenicity, even at laboratory scale. However, low process recovery has driven the practice of limiting the number of process steps, so purity is often sacrificed. The success rates of vector development will be affected by unwanted immunological effects on the outcomes of preclinical studies. The Nereus LentiHERO® can reduce HCP and double-stranded DNA contamination of LVV feedstocks without compromising recovery, time, or throughput (Figure 6).
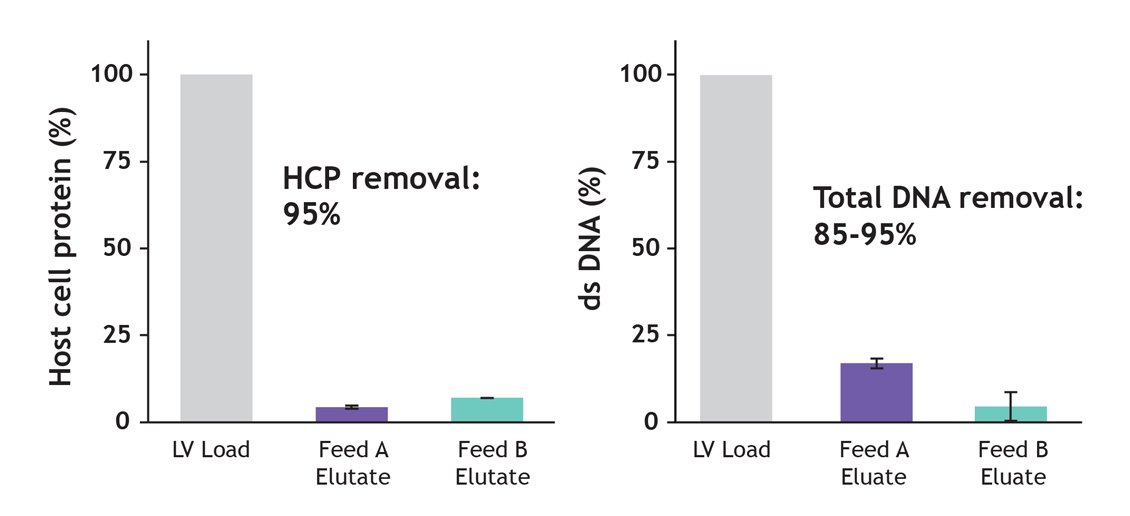
Figure 6: HEK293 host cell protein (HCP) levels were measured by ELISA, and total dsDNA by Picogreen assay. Feed B was treated by 5U/mL DENARASE® prior to clarification. LVV Load HCP concentration: Feed A , ~ 2μg/mL; Feed B, 0.8μg/mL. LVV load dsDNA concentration: Feed A, ~ 1.5μg/mL; Feed B, 0.5μg/mL.
Purified lentiviral particles retain functionality, morphology, and size
The structure and infectivity of purified LVV particles are uncompromised when they are processed with the Nereus LentiHERO®, thereby retaining both functionality and morphology. Figure 7 shows that the size of important proteins such as group-specific antigen (gag), encoding capsid, matrix, and nucleocapsid were unaffected. Likewise, the eluted LVV particle size and morphology were not influenced by purification with the Nereus LentiHERO® technology, and infectivity of the purified LVV particles was not compromised.
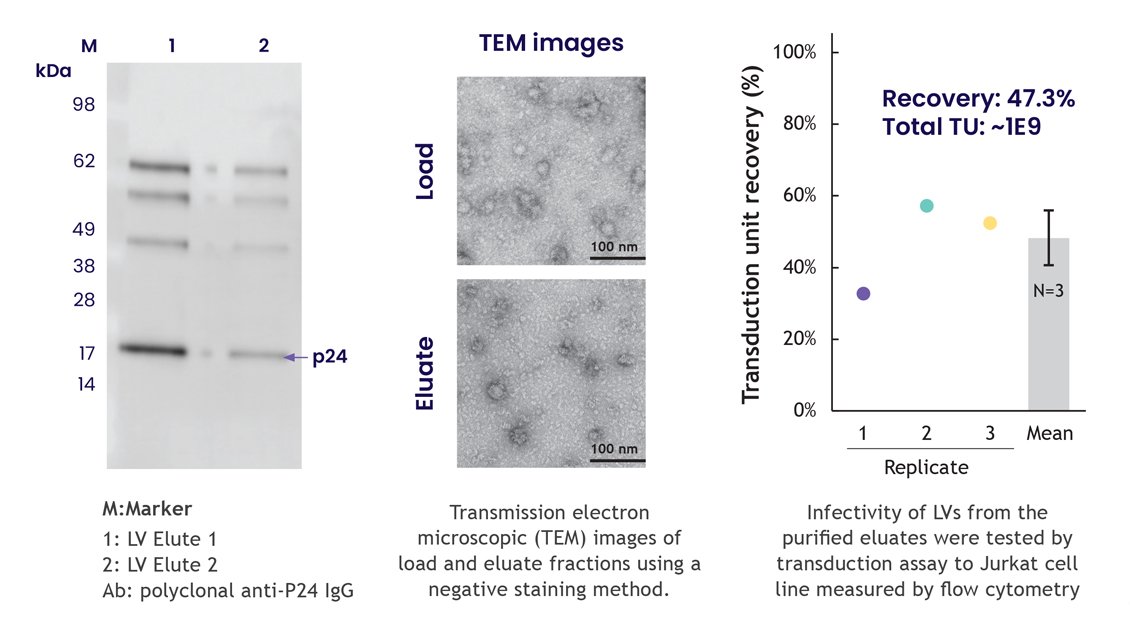
Figure 7: LVV particle structure and infectivity are not compromised after purification
The Nereus LentiHERO® – a lab scale solution for high yield, purity and accelerated processing
Reducing both contaminant levels and sample volumes facilitates subsequent concentration. The Nereus LentiHERO® spin-column format enables sample throughput to be increased easily by scaling out and maximizing benchtop centrifuge capacity. By incorporating the Nereus LentiHERO® solution into LVV feedstock preparation workflows, multiple samples can be processed simultaneously, and bottlenecks can be reduced.
The current options to address laboratory-scale LVV purification have limited capabilities for use with CGT modalities. To address the increasing demands on therapy developers, novel solutions that are fit-for purpose are essential. The Nereus LentiHERO® solution for LVV development and production laboratories is designed to increase sample-processing throughput and improve upon LVV particle recoveries. This nanofiber-based technology reduces bottlenecks in the development pipeline, ultimately helping the CGT industry to bring therapies to patients faster than ever before. In the future, Astrea Bioseparations plans to scale this technology, ultimately delivering larger fit-for-purposes devices to the market to ensure the successful scale up from lab scale to production.

