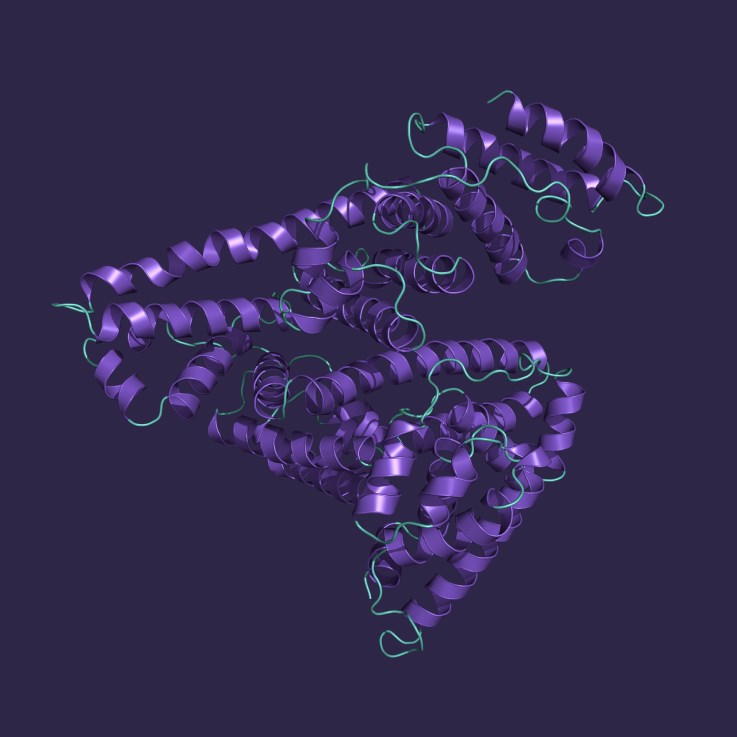
Serum albumin plays a crucial role in maintaining blood oncotic pressure and transporting various compounds throughout the body, including lipids, bile pigments, hormones, and metal ions. Mammalian albumins have many applications in research and diagnostics and human serum albumin (HSA) is produced in large quantities for treating conditions such as hypovolemic shock and hypoalbuminemia.
While HSA is traditionally produced from pooled human plasma using cold ethanol precipitation (the Cohn Process), chromatographic methods are increasingly being adopted either as an alternative or in addition to the Cohn Process to enhance purities and recoveries. Astrea Bioseparations supplies high-performance chromatography resins that are optimized for the capture and purification of serum albumin from various sources.
Albumin is not naturally glycosylated but is highly susceptible to non-enzymatic glycation. In therapeutics, albumin is purified from plasma for use as a stabilizer, drug carrier, and plasma volume expander, where its modification state is not typically separated or controlled. However, in diagnostics, glycated albumin has growing importance as a marker of short-term glycemic control, offering a complementary readout to HbA1c and providing valuable insights in clinical settings where hemoglobin-based testing is less reliable.